The U.S. Environmental Protection Agency (EPA) brought back its Climate Change Indicator’s platform last week. In EPA’s website page, titled “Climate Change Indicators: Ocean Acidity,” the agency tries to stoke fears human carbon dioxide emissions threaten to make the oceans acidic. This is untrue. As the data EPA cites actually shows oceans are and will remain alkaline under any reasonably expected increase in ocean carbon dioxide concentrations.
“Measurements made over the last few decades have demonstrated that ocean carbon dioxide levels have risen in response to increased carbon dioxide in the atmosphere, leading to an increase in acidity (that is, a decrease in pH),” writes EPA. “[R]ising levels of carbon dioxide dissolved in the ocean can have a negative effect on some marine life … mak[ing] it more difficult for these animals to thrive.”
The data EPA cites to make these claims support neither claim. Oceans are not in danger of becoming acidic, and rising carbon dioxide levels are, in fact, benefitting most sea life.
As Climate at a Glance: Ocean Acidification explains,
“A pH of 7 is considered neutral, with anything below 7 considered acidic. Ocean pH averages 8.1, which is alkaline rather than acidic. Although climate models suggest the oceans’ surface pH may have dropped from pH 8.2 to 8.1 since 1750, that change was never actually measured. The pH drop is merely a modeled conjecture.”
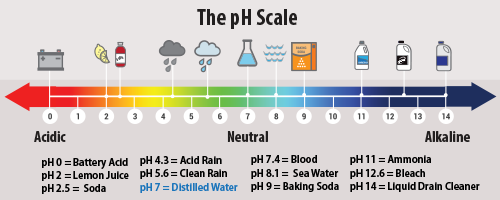
Note that sea water has a normal ph of 8.1 by the EPA’s own admission, and none of the highly magnified graphs illustrated on the new EPA website seen in figure 2 below get anywhere close to 7.0 or below, which is the acidic range on the pH scale. In fact, EPA doesn’t even show 7.0 on any of those graphs, making it misleading.
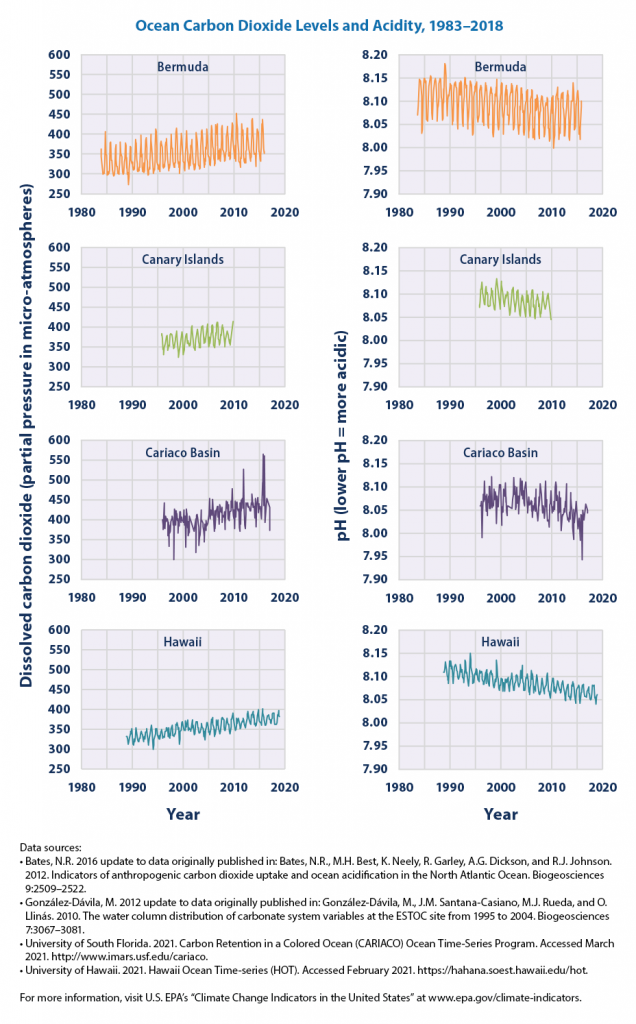
We are already more than halfway towards the feared doubling of carbon dioxide levels since the industrial revolution in 1850. Yet, according to climate model simulations, the pH of surface ocean waters has fallen by, at most, 0.1 pH units. This modest decline in pH is a long way from acidic. There is no reason for believing oceans will become acidic at the levels various climate models project carbon dioxide concentrations will peak, even under the most extreme scenarios.
Recent research from scientists at the CO2 Coalition, Ocean Health – Is there an “Acidification” problem?, documents ocean health improves rather than suffers with more carbon dioxide. Carbon Dioxide is food for phytoplankton that forms the foundation of the marine food chain. Also, studies show marine life thrive and enhance their growth rates in elevated CO2 conditions.
The oceans face a variety of problems EPA could discuss – from overfishing, to pollution, to localized eutrophication – but acidification is not one of them. This is another instance of the politicization of EPA under the Biden/Harris administration. The EPA’s Climate Change Indicator on ocean acidification ignores the agency’s own data, which shows no cause for alarm about ocean acidification, to hype imagined threats to sea life from supposed human caused global warming. What happened to following the science?























Mr. Burnett why in your article did you not state the pH is a logarithmic scale, so that a pH drop of 8.2 to 8.1 represents a 30% increase in the acidity of a liquid. If I permanently increased the temperature in your house by 30% would that have only ‘modest’ impact on your homeostasis I wonder? You must know that acidifying oceans does not mean that seawater on average will become acidic (i.e. less than pH of 7). Acidification just says the amount of hydrogen ions are increasing, which they are. That ‘EPA data’ is NOAA and and WMO long term monitoring stations which does clearly demonstrate increasing pCO2 concentrations and decreasing pH overtime. Those rates of change are only expected to increase, so that soon a 30% decrease in pH will become a 50% decrease and so forth, and that will indeed be a big deal for many kinds of sealife in our oceans.
If you crunch the numbers, you’ll find that the ocean is 13 times less acidic than distilled water.
Lol Trevor, I’ll answer your hypothetical. Because temperature is experienced arithmetically not logarithmically. Nice try though peppering in fancy words you don’t understand.